How LONSURF May Help
Clinical Trial Results
LONSURF has been proven to help some patients:
Live longer
Reduce the progression of cancer
Maintain the same level of functioning longer
SUNLIGHT Trial: LONSURF with Bevacizumab for Colon or Rectal Cancer
The SUNLIGHT clinical trial was a study of 492 patients that looked at the effectiveness and safety of LONSURF used together with bevacizumab compared to LONSURF alone for patients who had already received at least 2 types of treatment for their advanced colorectal cancer. The primary objective was overall survival. Some key secondary objectives were progression-free survival (PFS) and the time to deterioration of Eastern Cooperative Oncology Group Performance Status (ECOG PS).
Overall survival


Overall survival is the time from when the patient starts treatment until they pass away. Patients taking LONSURF with bevacizumab had a median overall survival of 10.8 months compared to 7.5 months in the patients receiving only LONSURF—a 3.3‑month improvement. That means half of the patients in the group that received LONSURF with bevacizumab lived less than 10.8 months but the other half lived longer than that.
Progression-free survival
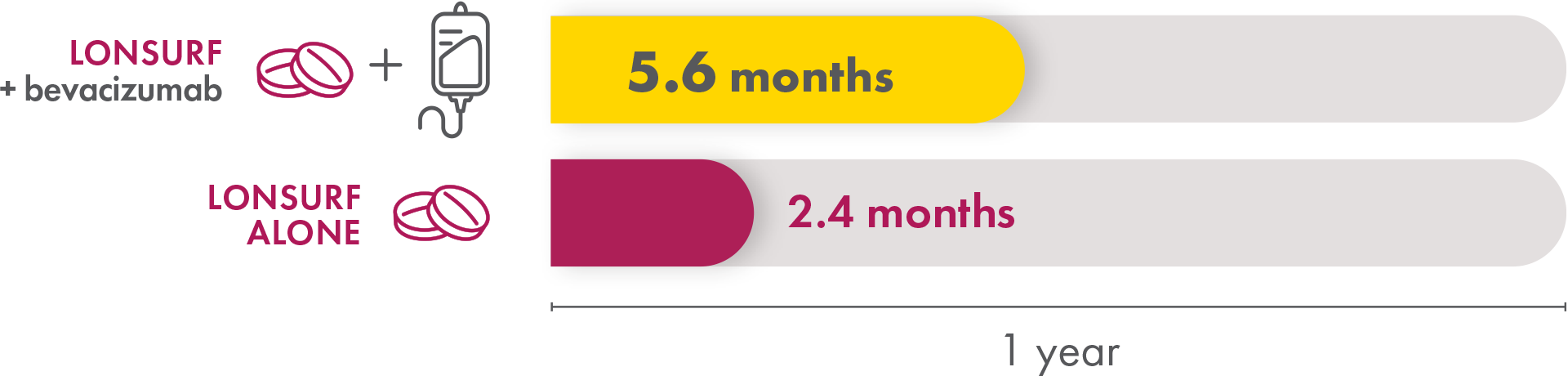

Progression-free survival is a measure of how long someone is on treatment before their cancer starts to grow. Patients taking LONSURF with bevacizumab had a median progression-free survival of 5.6 months compared to 2.4 months in the patients receiving only LONSURF—a 3.2‑month improvement.
Measuring daily function
ECOG Performance Status score is a way to measure how well a patient is functioning in daily life, including things like taking care of themselves, walking around, or working. On the scale, 0 means someone is fully active and has no restrictions while 5 means they have passed away. So, the lower your ECOG Performance Status score, the better.
| Score | ECOG Performance Status |
|---|---|
| 0 | Fully active |
| 1 | Ambulatory and able to carry out light work but restricted in strenuous activity |
| 2 | Bedbound less than 50% of waking hours and capable of self‑care |
| 3 | Bedbound more than 50% of waking hours and capable of limited self‑care |
| 4 | Bedbound, completely disabled and incapable of self‑care |
| 5 | Deceased |
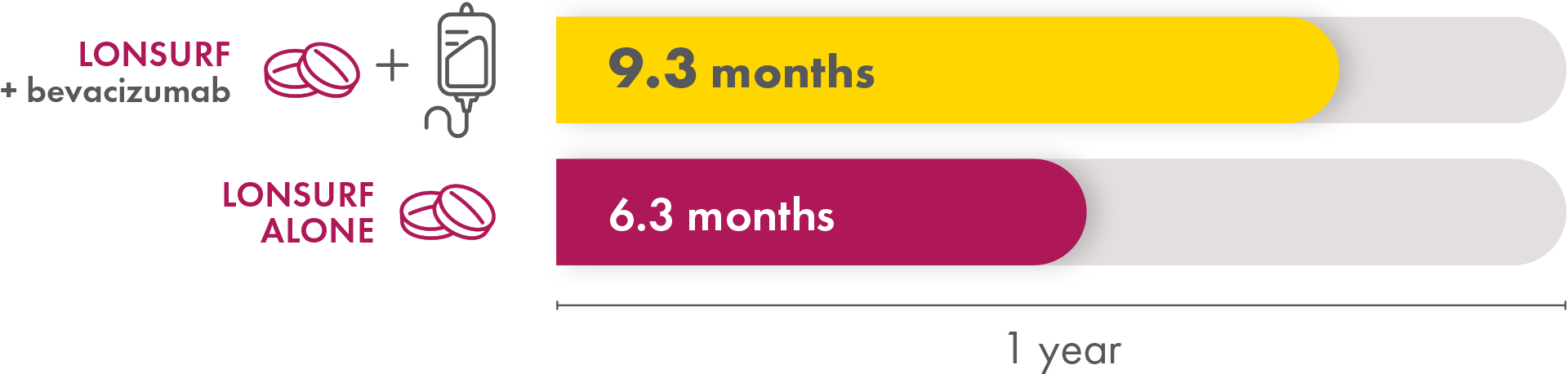
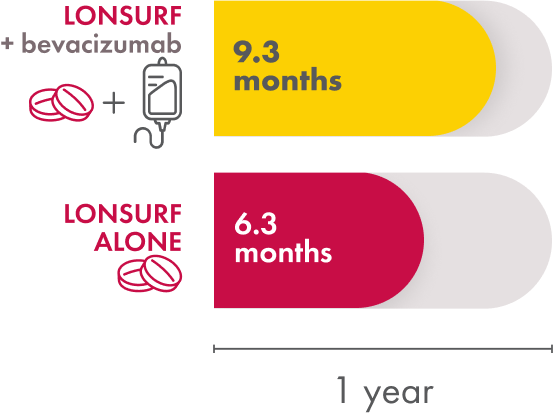
In the LONSURF + bevacizumab group, the median time to ECOG Performance Status score going from 0 or 1 to 2 or more was 9.3 months. The LONSURF-only group had a median time of 6.3 months. So patients who received LONSURF + bevacizumab stayed at a lower, better, ECOG Performance Status longer than patients who received only LONSURF.
RECOURSE Trial: Colorectal Cancer Treatment with LONSURF Alone
The RECOURSE clinical trial was a study of 800 patients that looked at the effectiveness and safety of LONSURF compared to placebo for patients who had already received at least 2 types of treatment for their advanced colorectal cancer. The primary objective was overall survival. Some key secondary objectives were progression-free survival (PFS) and the time to deterioration of Eastern Cooperative Oncology Group Performance Status (ECOG PS).
Overall survival

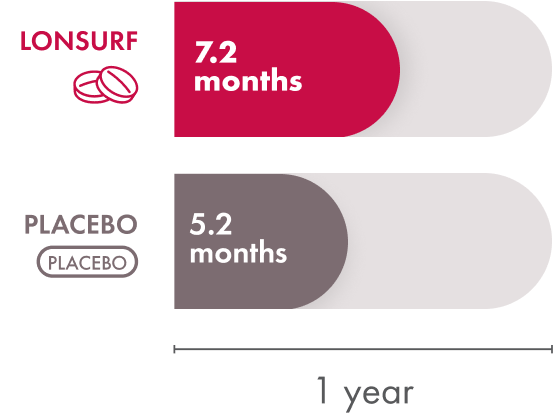
Overall survival is the time from when the patient starts treatment until they pass away. Patients taking LONSURF had a median overall survival of 7.2 months compared to 5.2 months in the patients receiving placebo—a 2‑month improvement. That means half of the patients in the group that received LONSURF lived less than 7.2 months but the other half lived longer than that.
Progression-free survival
Progression-free survival is a measure of how long someone is on treatment before their cancer starts to grow. Patients taking LONSURF had a median progression-free survival of 2.0 months compared to 1.7 months in the patients receiving placebo.
Measuring daily function
ECOG Performance Status score is a way to measure how well a patient is functioning in daily life, including things like taking care of themselves, walking around, or working. On the scale, 0 means someone is fully active and has no restrictions while 5 means they have passed away. So, the lower your ECOG Performance Status score, the better.
| Score | ECOG Performance Status |
|---|---|
| 0 | Fully active |
| 1 | Ambulatory and able to carry out light work but restricted in strenuous activity |
| 2 | Bedbound less than 50% of waking hours and capable of self‑care |
| 3 | Bedbound more than 50% of waking hours and capable of limited self‑care |
| 4 | Bedbound, completely disabled and incapable of self‑care |
| 5 | Deceased |

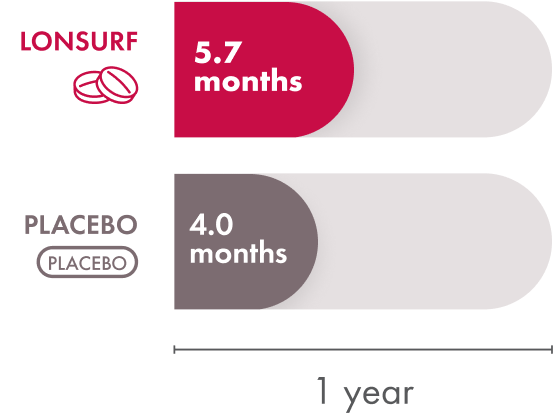
In the LONSURF group, the median time to ECOG Performance Status score going from 0 or 1 to 2 or more was 5.7 months. The placebo group had a median time of 4.0 months. So patients who received LONSURF stayed at a lower, better, ECOG Performance Status longer than patients who received the placebo.
TAGS Trial: Stomach Cancer Treatment with LONSURF
The TAGS clinical trial was a study of 507 patients that looked at the effectiveness and safety of LONSURF compared to placebo for patients who had already received at least 2 types of treatment for their advanced stomach cancer. The primary objective was overall survival. Some key secondary objectives were progression-free survival (PFS) and time to deterioration of Eastern Cooperative Oncology Group Performance Status (ECOG PS).
Overall survival
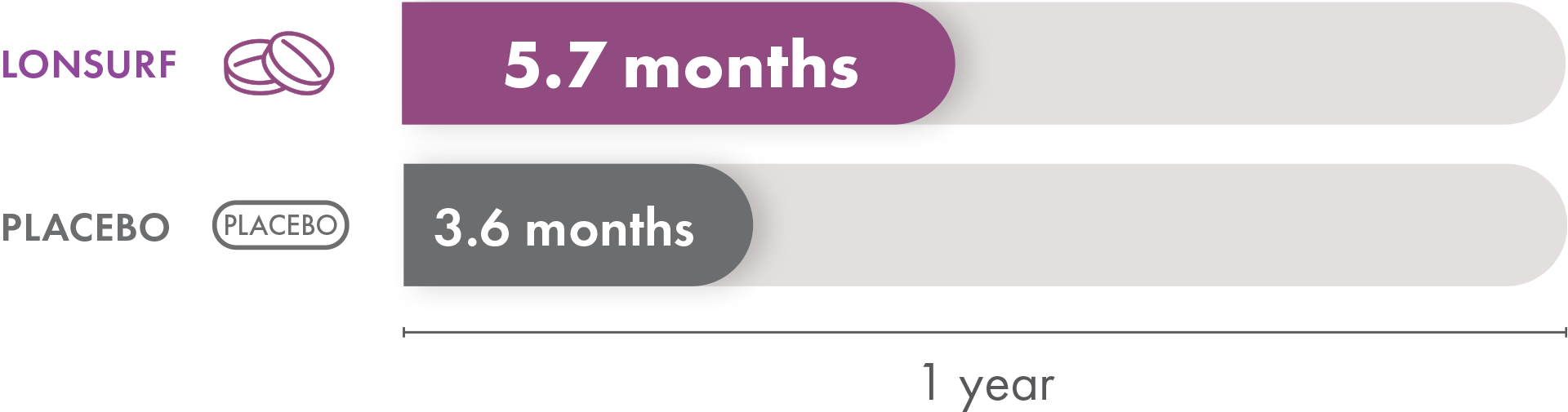
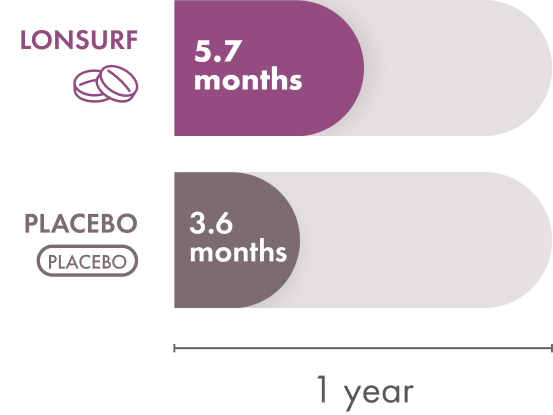
Overall survival is the time from when the patient starts treatment until they pass away. Patients taking LONSURF had a median overall survival of 5.7 months compared to 3.6 months in the patients receiving only placebo. That means half of the patients in the group that received LONSURF lived less than 5.7 months but the other half lived longer than that.
Progression-free survival
Progression-free survival is a measure of how long someone is on treatment before their cancer starts to grow. Patients taking LONSURF had a median progression-free survival of 2.0 months compared to 1.8 months in the patients receiving only placebo.
Measuring daily function
ECOG Performance Status score is a way to measure how well a patient is functioning in daily life, including things like taking care of themselves, walking around, or working. On the scale, 0 means someone is fully active and has no restrictions while 5 means they have passed away. So, the lower your ECOG Performance Status score, the better.
| Score | ECOG Performance Status |
|---|---|
| 0 | Fully active |
| 1 | Ambulatory and able to carry out light work but restricted in strenuous activity |
| 2 | Bedbound less than 50% of waking hours and capable of self‑care |
| 3 | Bedbound more than 50% of waking hours and capable of limited self‑care |
| 4 | Bedbound, completely disabled and incapable of self‑care |
| 5 | Deceased |
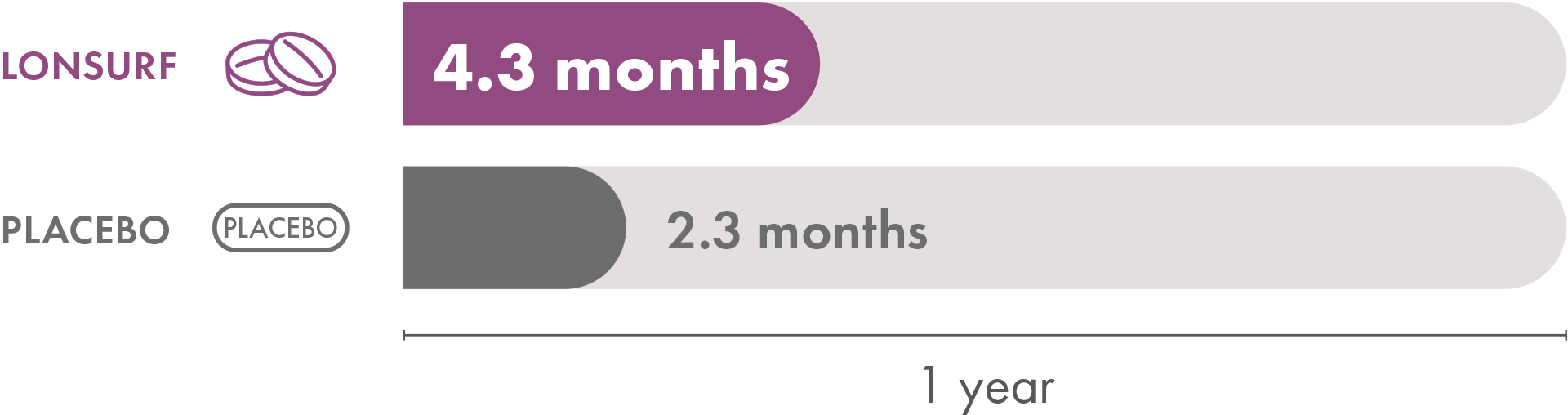
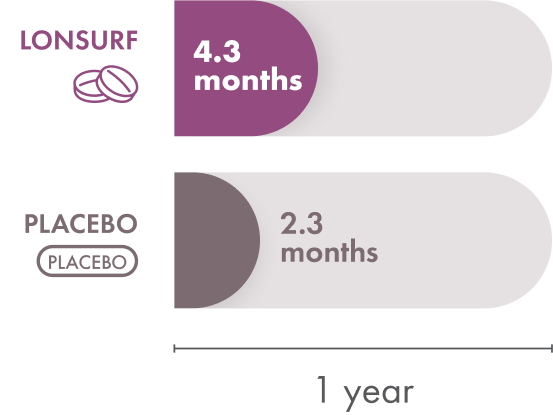
In the LONSURF group, the median time to ECOG Performance Status score going from 0 or 1 to 2 or more was 4.3 months. The placebo group had a median time of 2.3 months. So patients who received LONSURF alone stayed at a lower, better, ECOG Performance Status longer than patients who received placebo.
How it Works
LONSURF consists of two medicines in the same tablet
Trifluridine may help prevent tumor growth by interfering with the tumor's DNA
Tipiracil helps trifluridine work longer
(also referred to by the brand name Avastin®), which may help slow or stop blood supply to the tumor. You may have previously received bevacizumab as part of an earlier line of therapy.
INDICATIONS
INDICATIONS
LONSURF is a prescription medicine used:
- alone or in combination with the medicine bevacizumab to treat adults with colorectal cancer:
- that has spread to other parts of the body, and
- who have been previously treated with certain chemotherapy medicines
- alone to treat adults with a kind of stomach cancer called gastric cancer including adenocarcinoma of the gastroesophageal junction:
- that has spread to other parts of the body, and
- who have been previously treated with at least 2 types of treatment which included certain medicines
It is not known if LONSURF is safe and effective in children.


INDICATIONS
LONSURF is a prescription medicine used:
- alone or in combination with the medicine bevacizumab to treat adults with colorectal cancer:
- that has spread to other parts of the body, and
- who have been previously treated with certain chemotherapy medicines
- alone to treat adults with a kind of stomach cancer called gastric cancer including adenocarcinoma of the gastroesophageal junction:
- that has spread to other parts of the body, and
- who have been previously treated with at least 2 types of treatment which included certain medicines
It is not known if LONSURF is safe and effective in children.
IMPORTANT SAFETY INFORMATION
LONSURF may cause serious side effects, including:
- Low blood counts. Low blood counts are common with LONSURF and can sometimes be severe and life-threatening. LONSURF can cause a decrease in your white blood cells, red blood cells, and platelets. Low white blood cells can make you more likely to get serious infections that could lead to death. Your healthcare provider should do blood tests before you receive LONSURF, at day 15 during treatment with LONSURF, and as needed to check your blood cell counts. Your healthcare provider may lower your dose of LONSURF or stop LONSURF if you have low white blood cell or platelet counts
Tell your healthcare provider right away if you get any of the following signs and symptoms of infection during treatment with LONSURF: fever, chills, or body aches.
Before taking LONSURF, tell your healthcare provider about all of your medical conditions, including if you:
- Have kidney or liver problems
- Are pregnant or plan to become pregnant. LONSURF can harm your unborn baby
- Females who can become pregnant: Your healthcare provider will do a pregnancy test before you start treatment with LONSURF. You should use effective birth control during and 6 months after the last dose of treatment with LONSURF. Tell your healthcare provider immediately if you become pregnant
- Males, while on treatment and for 3 months after your last dose of LONSURF, you should use a condom during sex with female partners who are able to become pregnant. Tell your healthcare provider right away if your partner becomes pregnant while you are taking LONSURF
- Are breastfeeding or plan to breastfeed. It is not known if LONSURF passes into your breast milk. Do not breastfeed during treatment with LONSURF and for 1 day after your last dose of LONSURF
Tell your healthcare provider about all the prescription and over-the-counter medicines, vitamins, and herbal supplements you take.
The most common side effects of LONSURF when used alone include low blood counts, tiredness and weakness, nausea, decreased appetite, diarrhea, vomiting, stomach-area (abdominal) pain, and fever.
The most common side effects of LONSURF when used in combination with bevacizumab include low blood counts, tiredness and weakness, nausea, certain abnormal liver function blood tests, decreased salt (sodium) in your blood, diarrhea, stomach-area (abdominal) pain, and decreased appetite.
Tell your healthcare provider if you have nausea, vomiting, or diarrhea that is severe or that does not go away.
These are not all of the possible side effects of LONSURF. For more information, ask your healthcare provider. Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.



